| Back to . . . .
 Curve Bank Home Page Curve Bank Home Page
NCB
Deposit # 28
William A. Hoffman
Department of Chemistry
Denison University
Granville, Ohio
hoffman@denison.edu
|

Diprotic Acid-Base Equilibria
Two Protons in Action!
|
| The graphics in this deposit were created using Maple software

and were suggested to the NCB by
Jason Schattman, Ph.D.
Applications Marketing Manager
Waterloo Maple, Inc.
|
|
| Background
for the student . . . . .
Diprotic
acids are
substances (molecules) that have two protons that can be released in
water.
A very common diprotic acid is sulfuric acid, H2SO4.
In
water, both protons of sulfuric acid are released, giving hydrated
protons 2H+(aq)
and sulfate dianion, SO4 (2-):

The pH of the solution, since it is very acidic, is low. (The number is well below zero!)
Carbonated water (club soda) is another example of a diprotic acid. This diprotic acid and its monoprotic acid partner are formed when carbon dioxide is bubbled through water.

In a
general case as
the pH of an aqueous solution increases, a diprotic acid will ionize
first
to a monoprotic acid ( ha- )
and one proton ( H+ ) and then the monoprotic acid
( ha- )
will ionize to a dianion ( a= ) and
liberate the second proton ( H+ ).
|

|
| Study the graph below. Think about what is happening. As one curve decreases, what is happening to the other curve(s)? Many students find it easier to remember a reaction if he or she actually makes a sketch of the graph. |
 |
|

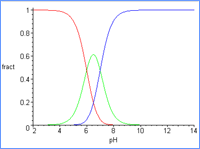
As the
pH increases (horizontal axis), the fraction of the diprotic acid (
yellow
curve ) decreases while the fraction of the
monoprotic anion ( green curve )
increases. Then, above pH = 8, the dianion ( red
curve representing no protons ) forms as the monoprotic
acid
(green curve) is depleted.

|
Study the curves on the left (below) and then click on the graph to see the animation. These exercises use examples of acids found in chemical and biochemical systems.
|

This animation demonstrates what happens when the value of k2 changes relative to k1.
Changing values of k1, k2 - or
the pH range -
show the effect these variables have on the fraction of each species
present.
Even when k2 = k1 (unlikely) there is a
small
amount of (ha-)
present.
|
|
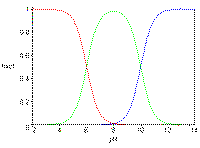
In this
animation, study
the bar moving across the graph.
The
pH "bar"
is animated by half -pH
units which allows one to follow more accurately the fraction of
diprotic
(h2a), monoprotic (ha-), and
aprotic
(a=) species present at each pH
value.
|
|
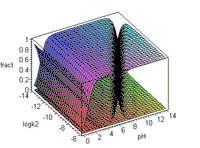
k1 = 10-9.... 10-1 (varies)
k2 = 10-14.... 10-6 (varies) |

The 3d animation of k1, k2, and pH allows an overview of
the way fractions
of the species (h2a),
(ha-), and (a=)
predominate at different pH levels. When pH > 9, ( a=)
will predominate unless k2 approaches 10-14.
Conversely, at pH = 0, if k1 = 10-1,
the fraction of h2a is still less than 1. Under most aqueous
solution
conditions, the (ha-)
fraction
will be significant. Note the limits placed on k1 and k2 in this
illustration. |
|
Dr. Arnold O. Beckman, Inventor of the pH Meter
Any National Curve Bank deposit on acid-base reactions would not be complete without acknowledging Dr. Arnold O. Beckman, the inventor of the pH meter and a major contributor to the technology revolution of the 20th century.
This
photo of Dr.Beckman
was taken on his 100th birthday, April 10, 2000, at Casa Pacifica, San
Clemente, California. In addition to his many other
accomplishments -
scholar, inventor, civic leader and philanthropist -
he is one of the U.S.'s oldest living Marines.
Arnold
has several
rules for living a good life. One is always do your best.
Never
do anything half-heartedly.
|
 |
| Zaven Karian at Denison University in Granville, Ohio brought together faculty from across liberal arts departments to see what they might do with computational tools using Maple software. Hoffman's pH animations are one outcome. The project was supported by the Fund for Improvement of Post Secondary Education (FIPSE) of the Department of Education (Grant #P116B30079).
The NCB thanks Hoffman and Karian for their fine efforts. Moreover, we thank Jason Schattman of Waterloo Maple, Inc. for calling this work to our attention. This deposit represents a merger of applied mathematics with chemistry using computer software. |
| For the Maple code that created the animations please see < http://www.mapleapps.com/categories/science/chemistry/html/Ab6.html > |
|